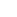CDMO Services for Lentiviral Vectors - IND grade
Services
| IND grade | 1 | Independently developed four-plasmid system |
● Third generation four-plasmid system ● Kanamycin-resistance gene ● Granting the license, if required |
● Following standards for submission in both China and US ● Full-GMP workshop ● Separate area for creating cell banks ● Separate workshops within non-sterile and sterile areas ● GMP quality management system |
| 2 | Creation of GMP Cell Bank |
● Tailorable number of cell banks to be created ● Cell bank stability study |
||
| 3 | Process and Test Method Development |
● Following project requirements (subject to customized changes) |
||
| 4 | GMP Manufacturing of Lentiviral Vectors |
● Bioreactor process: 5~50 L disposable bioreactor process (subject to customized changes) ● Production scale: 2~30 L (subject to customized changes) |
||
| 5 | Testing of Lentiviral Vectors |
● Physical titer ● Infective titer ● Functional titer ● Residual 293T host cell DNA testing ● Residual 293T host cell protein testing ● Residual exogenous DNA testing ● Residual Benzonase testing ● E1A/SV40 ● Residual plasmid testing ● DNA fragment size ● Exogenous virokines ● Sterility ● Mycoplasma ● Endotoxin |
||
| 6 | Method Validation |
● Specificity ● Accuracy ● Precision ● Linearity and Range ● LOD |
||
| 7 | Stability Study |
● Long-term stability ● Accelerated stability ● Stress testing |
Advantages
| Advantages of using our platform for serum-free suspension culturing of lentiviral vectors:
• Free of animal-derived components throughout the process • Linearly scaled up production of lentiviral vectors • Using a single container of a 50 L disposable bioreactor • Cell bank creation in separate workshops • Dispensing final products using a sterile isolator • Dedicated lentivirus system for CAR-T cells, with high infection efficiency • Low production costs and testing costs (no requirements of testing for BSA and residual pancreatic enzymes) • Several successful IND submissions to NMPA of lentiviral vectors for CAR-T cells |
Manufacturing process
Quality control
| Product | Test Item | Test Method |
| Harvest Fluid | Adventitious virus contamination | Method 3302 of ChP 2020 |
| Replication-competent lentiviruses | Indicator cell culture method | |
| Drug substance/finished product | Appearance | Visual inspection |
| Sterility | Method 1101 of ChP 2020 | |
| Mycoplasma |
Method 3301 of ChP 2020 |
|
| pH | Method 0631 of ChP 2020 | |
| Osmolality | Method 0632 of ChP 2020 | |
| Target gene structure identification | Sequencing | |
| Residual host cell protein | ELISA | |
| Physical titer (p24) | ELISA | |
| Functional titer | Flow cytometry | |
| Endotoxin | Method 1143 of ChP 2020 | |
| Residual Benzonase | ELISA | |
| Residual host cell DNA | q-PCR | |
| Residual E1A gene transfer | Co-culture method | |
| Residual SV40 gene transfer | Co-culture method |
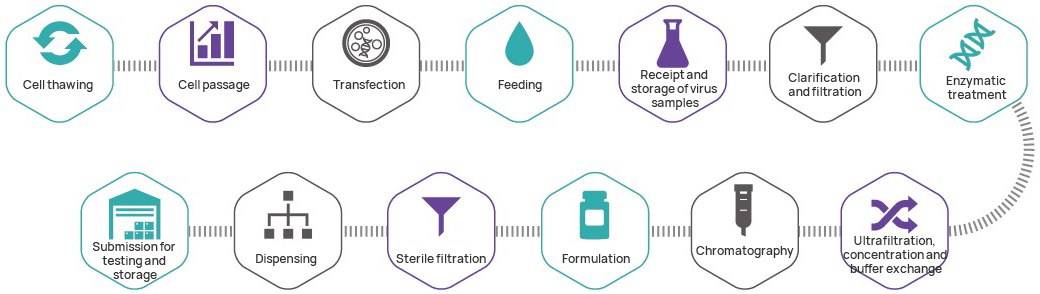
Project Timeline
Project Management Plan
Hillgene Project Management Team, consisting of chief scientists, project managers, Project QA and GMP experts, will make efforts to ensure the smooth and sound operation of each and every GMP project.