CDMO Services for Plasmids-IIT grade/non-clinical phase
Services
| CDMO Services for Plasmids | ||||
| Types | Services | |||
| IIT grade | 1 | Independently developed four-plasmid system |
● Third generation four-plasmid system ● Kanamycin-resistance gene ● No patent license required |
● Seamless connection to IND submission ● GMP-like workshop ● GMP-like quality management system ● Authentic and traceable documentation |
| 2 | Creation of bacteria bank (GMP-like) |
● Tailorable number and specification of bacteria banks to be created |
||
| 3 | Plasmid Manufacturing and Testing (GMP-like) |
● Tailorable production output and specification |
||
Advantages
| Advantages of our plasmid system:
• An independently developed four-plasmid system with kanamycin-resistance gene • A system with the capability of sustained optimization • Plasmid sequences are traceable, compliant with requirements, and efficient • Extensive experience in successful IND submissions • CAR-T cell samples for clinical use are currently manufacturing and in use • 2-5 folds higher titers after using our plasmid system from the comparison in several projects |
Advantages of our plasmid manufacturing: • Free of antibiotics throughout the manufacturing process • Plasmid production and bank creation in separate workshops • Complete isolation between non-sterile and sterile areas • Dispensing final products using an isolator • Completed CTD dossiers for packaging plasmid (for lentiviral vector), reducing the submission preparation time by 3-4 months, with INDs of a few products granted preliminary approval and currently in phase I of clinical study |
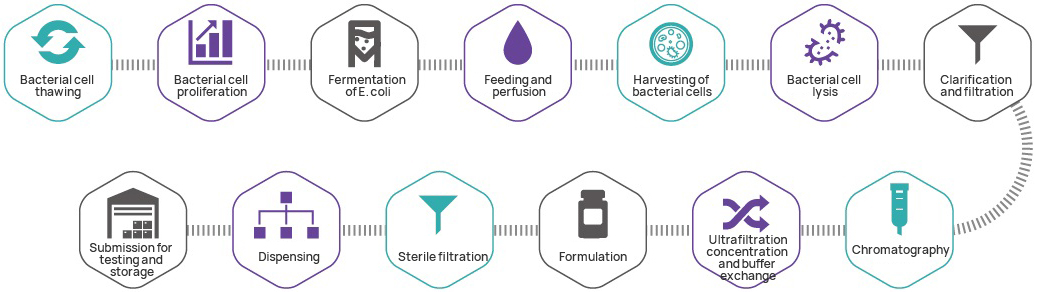
Manufacturing process
Quality control
| Test Item | Test Method | |
| Appearance | Visual inspection | |
| Identification | Identification 1 | Restriction mapping |
| Identification 2 | Sanger sequencing | |
| Test | pH | Method 0631 of ChP 2020 |
| Purity | High performance liquid chromatography (HPLC) | |
| Residual E.coli host cell protein | ELISA | |
| Residual E.coli DNA | q-PCR | |
| Residual E.coli RNA | q-PCR | |
|
Residual antibiotics |
ELISA | |
| Endotoxin | Method 1143 of ChP 2020 | |
| Sterility | Method 1101 of ChP 2020 | |
| Concentration determination | DNA concentration | Method 0401 of ChP 2020 |
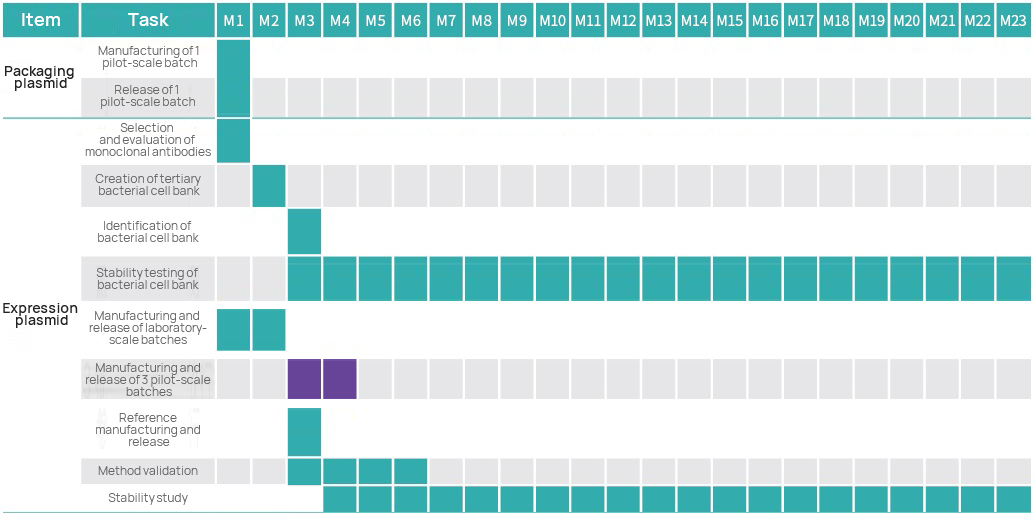
Project Timeline
Project Management Plan
Hillgene Project Management Team, consisting of chief scientists, project managers, Project QA and GMP experts, will make efforts to ensure the smooth and sound operation of each and every GMP project.