Vero Residual DNA Kit - Precise Mycoplasma Detection | BlueKit
Vero Residual DNA Kit - Precise Mycoplasma Detection | BlueKit
$ {{single.sale_price}}
In the realms of molecular biology and biopharmaceutical development, the accuracy and reliability of detecting contaminating DNA within cultures, such as Mycoplasma, can significantly influence the outcome of research and product safety. BlueKit’s Vero Residual DNA Kit - ZY002 has been meticulously designed to address this critical need, ensuring that scientists and researchers have access to a method that not only enhances the sensitivity of their analyses but also streamlines their workflow.
This comprehensive detection kit offers a robust solution for the precise identification and quantification of Vero cell residual DNA, a common challenge in the production of vaccines and biologics. Utilizing advanced qPCR technology, the kit provides rapid and reliable results, essential for maintaining the highest standards of quality control and regulatory compliance. With enough reagents for 50 reactions, the kit is perfectly suited to both high-throughput screens and specialized investigations, ensuring that regardless of the scale, your research can proceed with confidence. Beyond its technical capabilities, the Vero Residual DNA Kit underscores BlueKit’s commitment to supporting the scientific community in overcoming the challenges of contamination detection. The kit is accompanied by comprehensive instructions and expert support, ensuring that even laboratories new to qPCR can achieve accurate and reproducible results. Dive deeper into your analysis with a solution designed for excellence, and let BlueKit’s Vero Residual DNA Kit be your partner in advancing the frontiers of biopharmaceutical research and molecular diagnostics.
|
Specification
|
50 Reactions.
|
Standard curve
|
|
Datasheet
|
This comprehensive detection kit offers a robust solution for the precise identification and quantification of Vero cell residual DNA, a common challenge in the production of vaccines and biologics. Utilizing advanced qPCR technology, the kit provides rapid and reliable results, essential for maintaining the highest standards of quality control and regulatory compliance. With enough reagents for 50 reactions, the kit is perfectly suited to both high-throughput screens and specialized investigations, ensuring that regardless of the scale, your research can proceed with confidence. Beyond its technical capabilities, the Vero Residual DNA Kit underscores BlueKit’s commitment to supporting the scientific community in overcoming the challenges of contamination detection. The kit is accompanied by comprehensive instructions and expert support, ensuring that even laboratories new to qPCR can achieve accurate and reproducible results. Dive deeper into your analysis with a solution designed for excellence, and let BlueKit’s Vero Residual DNA Kit be your partner in advancing the frontiers of biopharmaceutical research and molecular diagnostics.
{{item.c_type}}
{{item.title}}
{{item.c_time_limit}}
{{item.title}}
Number
Overview
Protocols
Specifications
Shipping & Returns
Video Recording
Cat.No. HG-ZY002 $1,508.00
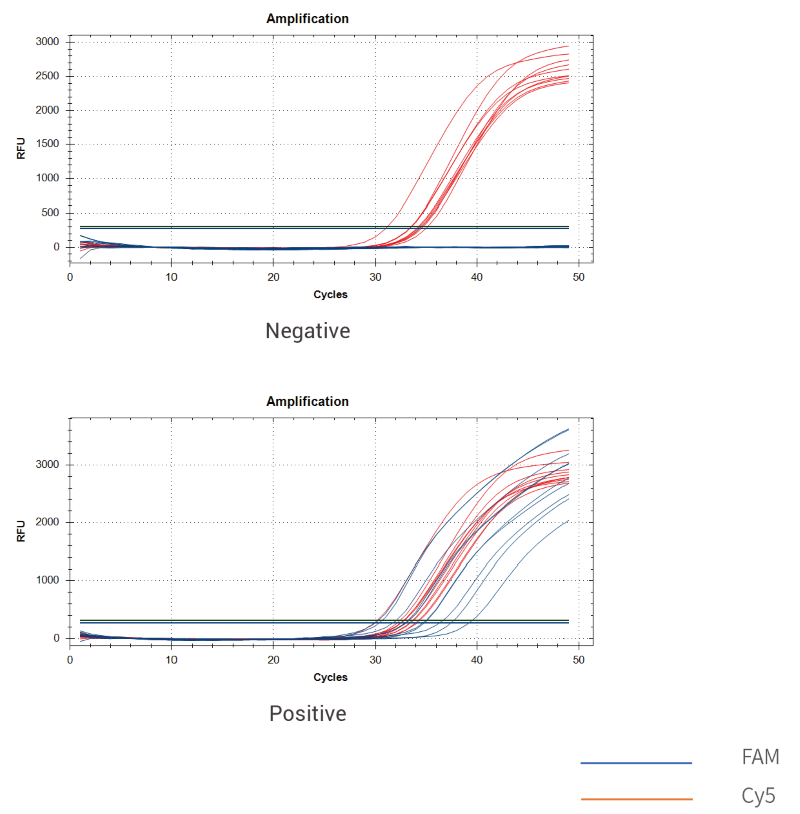
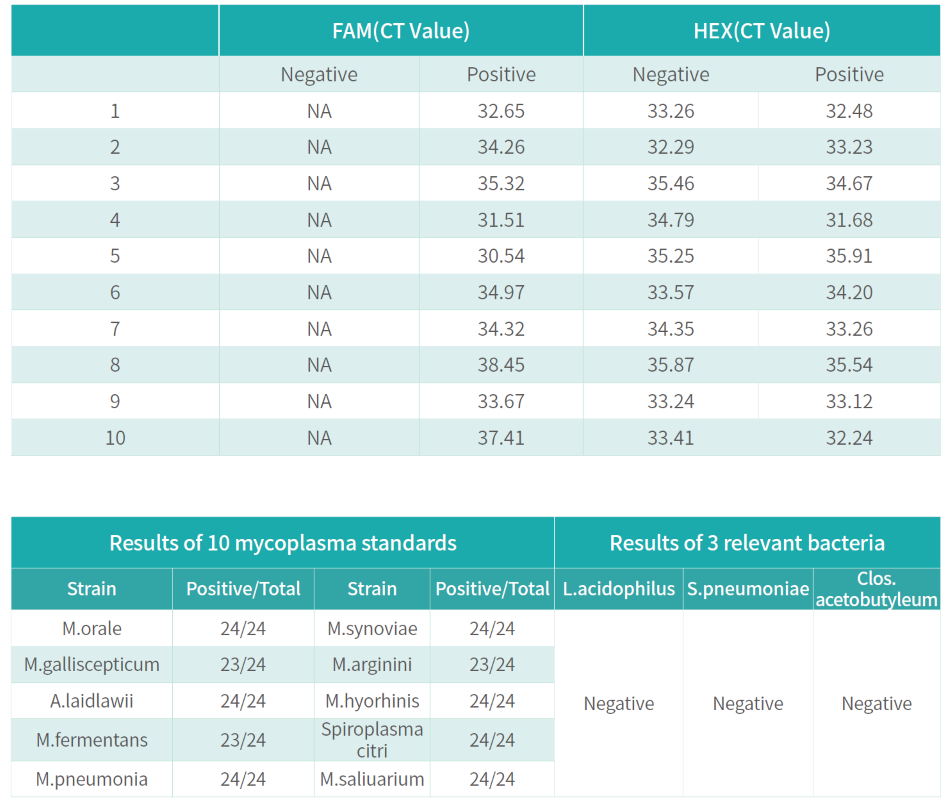
The kit is used to qualitatively detect the presence of mycoplasma contamination in master cell banks, working cell banks, virus seed lots, control cells, and cells for clinical therapy.
The kit uses qPCR-fluorescent probe technology to verify with reference to mycoplasma detectionrelated requirements in EP2.6.7 and JPXVII. It can cover more than 100 mycoplasmas and has no cross reaction with closely related strains. The detection is rapid which can be completed within 2 hours, with strong specificity.