Precision Vero Residual DNA Detection Kit - qPCR Analysis
Precision Vero Residual DNA Detection Kit - qPCR Analysis
$ {{single.sale_price}}
In the rapidly evolving landscape of biotechnological advancements, precise quantification of Vero cell residual DNA in biopharmaceutical products is pivotal. BlueKit proudly introduces the Vero Residual DNA Detection Kit, employing state-of-the-art quantitative PCR (qPCR) technology. This kit stands as a testament to our commitment to excellence, ensuring unparalleled accuracy and reliability in detecting and quantifying residual DNA.
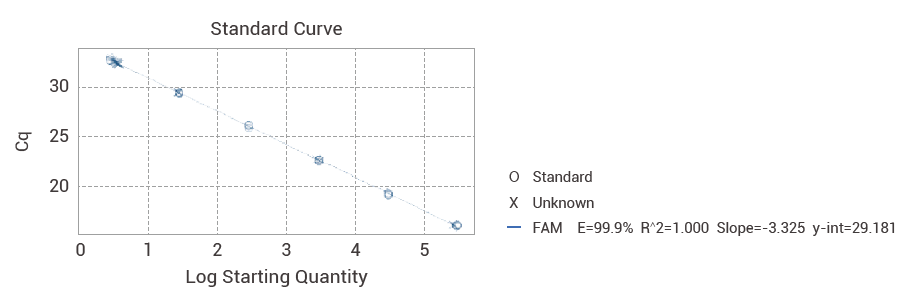
Our kit is meticulously designed to cater to the stringent requirements of quality control departments across the biopharma industry. It provides a robust, sensitive, and specific solution for monitoring Vero cell line DNA contaminants. The cornerstone of our kit is its precision-engineered standard curve, which ensures linear quantification across a broad dynamic range. This imparts the confidence needed to comply with regulatory standards, safeguarding the purity and safety of your biopharmaceutical products. The essence of the Vero Residual DNA Detection Kit lies in its user-centric design. It is accompanied by a comprehensive datasheet, detailing easy-to-follow protocols and ensuring seamless integration into existing laboratory workflows. The kit is not merely a product; it embodies our dedication to supporting the biopharmaceutical sector's commitment to quality and safety. With every test, it reinforces the trust placed in us by professionals seeking accuracy, efficiency, and reliability in Vero cell DNA quantification.
|
Standard curve
|
|
Datasheet
|
Our kit is meticulously designed to cater to the stringent requirements of quality control departments across the biopharma industry. It provides a robust, sensitive, and specific solution for monitoring Vero cell line DNA contaminants. The cornerstone of our kit is its precision-engineered standard curve, which ensures linear quantification across a broad dynamic range. This imparts the confidence needed to comply with regulatory standards, safeguarding the purity and safety of your biopharmaceutical products. The essence of the Vero Residual DNA Detection Kit lies in its user-centric design. It is accompanied by a comprehensive datasheet, detailing easy-to-follow protocols and ensuring seamless integration into existing laboratory workflows. The kit is not merely a product; it embodies our dedication to supporting the biopharmaceutical sector's commitment to quality and safety. With every test, it reinforces the trust placed in us by professionals seeking accuracy, efficiency, and reliability in Vero cell DNA quantification.
{{item.c_type}}
{{item.title}}
{{item.c_time_limit}}
{{item.title}}
Number
Overview
Protocols
Specifications
Shipping & Returns
Video Recording
Cat.No. HG-VE001 $1,692.00
This kit is designed for the quantitative detection of residual Vero host cell DNA in intermediates, semi-finished products and finished products of various biological products.
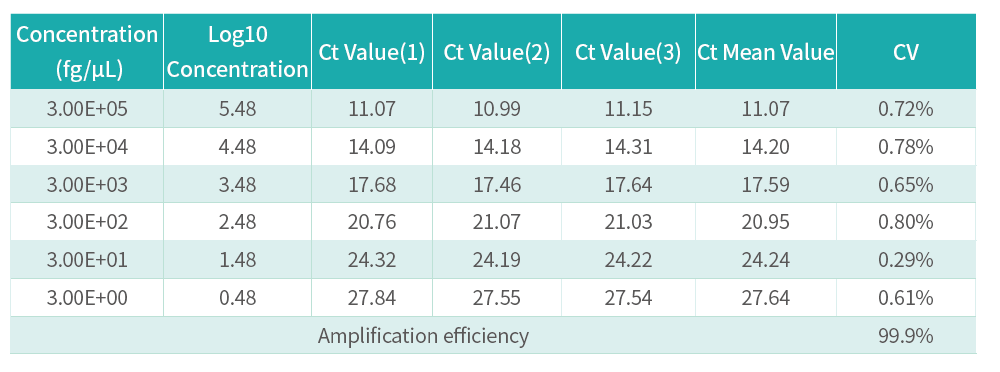
This kit adopts the principle of Taqman probe to quantitatively detect residual Vero DNA in samples. The kit is a rapid, specific and reliable device, with the minimum detection limit reaching fg level.
| Performance |
Assay range |
|
|
Limit of quantitation |
|
|
|
Precision |
|