CDmo Services ad Plasmids - Volume Grade
SERVITIUM
| CDmo Services ad Plasmids | ||||
| Genimen | SERVITIUM | |||
| C. Grade | 1 | GMP manufacturing of Plasmids |
● productio output: X mg ~ I g (sub ut customized mutationes) ● Fermentation Volume: III ~ XXX L (Subject ut customized mutationes) ● Purificacionis Methodo: Tres - Step Accede / Duo - Gradus Aditus |
● Full - GMP OFFICING ● Separatum Workshops intra non - sterilis et sterilis areas ● GMP qualitas Management System ● Validated plant, facilitas et apparatu obsequium cum orci requisitis |
| 2 | Technology Transfer |
● Technology translatio ● accipiens technology translationis |
● Bene - Statutum Plan Technology Transfer ● Bene - statutum consilium pro accepto technology translationis ● consilium transferendo diversis technologies trans diversis augmentis |
|
Commoda
| Commoda nostra Plasmid System:
• An independently developed quatuor - Plasmid ratio cum Kanamycin - Resistance Gene • A ratio cum facultatem sustentari Optimization • Plasmid sequentia sunt traceabilem, obsequium cum requisitis, et agentibus • extensive experientia in prospere ind penminesions • car - T cellula exemplaria ad orci usum sunt currently vestibulum et in usu • II - V Suspendisse altius titers post usura nostrum plasmid ratio ex comparatione in pluribus projects |
Commoda nostra Plasmid vestibulum: • liberabo de antibiotics per vestibulum processus • Plasmid productio et ripam creaturae in separatum officinam • Complete seorsum inter non - sterilis et sterilis areas • Dispensing Final Products Using an Isolator • Completur CTD dossiers ad packaging Plasmid (quia lentiviral vector), reducendo submission praeparatio tempus per III - IV menses, cum a paucis products dato premoneribus et in currently in tempore et in orci studio |
Processu
Imperium
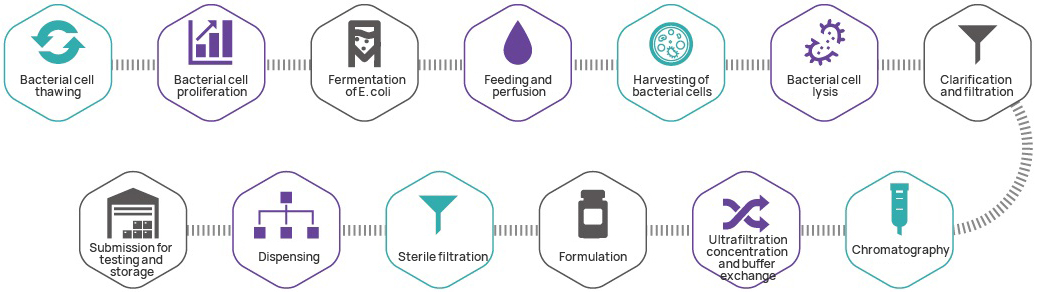
| Test item | Test modus | |
| Species | Visual inspectionem | |
| Idem | Idem I | Restricx Mapping |
| Identificatio II | Sanger sequencing | |
| Probo | pH | Modus DCXXXI de CHP MMXX |
| Pudicitia | High euismod Liquid Chromatography (HPLC) | |
| RELICTUM E.Coli exercitum cell dapibus | Elisa | |
| RELICTUM E.Coli DNA | Q - PCR | |
| Rena RNA RNA | Q - PCR | |
|
RELICTUM antibiotics |
Elisa | |
| Endotoxin | Modus MCXLIII de CHP MMXX | |
| Sterilitas | Modus MCI de CHP MMXX | |
| Concentration determinatio | DNA concentration | Modus MCCI de CHP MMXX |
Project Timeline
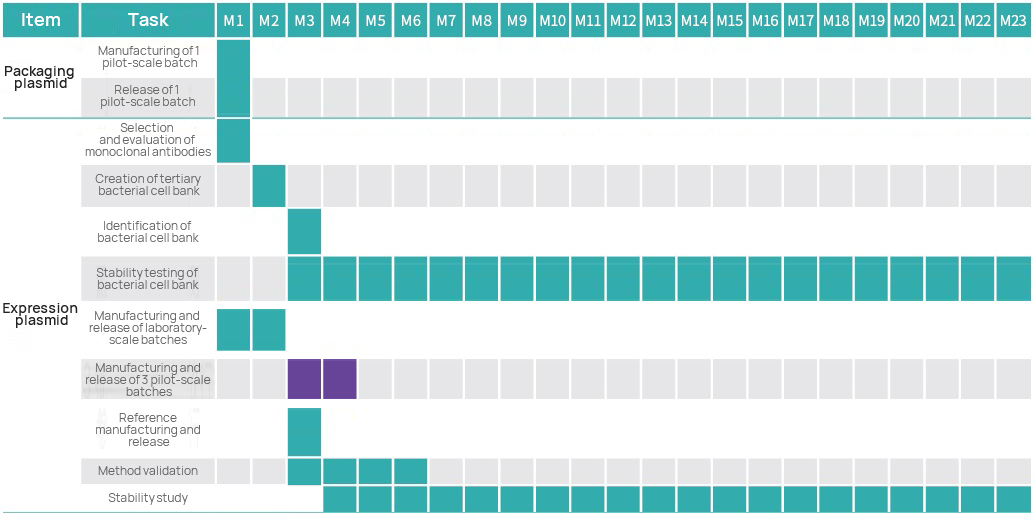
Project Management Plan
Hebgene Project Management Team, constans chishistae, project managers, project QA et GMP peritorum, et faciet studium ut lenis et sonus operationem et singulis et omni GMP project.