CHO DNA Detection Kit - Precise & Efficient Residual Testing
CHO DNA Detection Kit - Precise & Efficient Residual Testing
$ {{single.sale_price}}
In the realm of biopharmaceutical research and manufacturing, the necessity for precise, reliable detection of residual DNA has never been more critical. BlueKit is proud to introduce the forefront of molecular diagnostics: the Mycoplasma DNA Detection Kit (qPCR)-ZY002, your trusted partner in CHO DNA, CHO DNA Kit, CHO DNA Detection, and CHO Residual DNA Kit testing. Designed meticulously to meet the rigorous demands of modern laboratories, this kit marks a significant leap in the detection of CHO residual DNA, ensuring the integrity and safety of your bioproducts.
Our innovative kit, offering compatibility with qPCR platforms, comes with 50 reactions per package, providing not just unparalleled accuracy but also cost-effectiveness for laboratories of all sizes. The Mycoplasma DNA Detection Kit (qPCR)-ZY002 is tailored for those who seek efficiency without compromising on precision. Whether for routine quality control, research, or development purposes, this kit delivers consistent results, ensuring that your products meet the stringent standards for CHO residual DNA contamination. Embedded within this product is the essence of BlueKit's dedication to supporting the biopharmaceutical industry's push towards safer, more reliable products. By choosing the Mycoplasma DNA Detection Kit (qPCR)-ZY002, you're not just acquiring a product; you're empowering your research and production with a tool designed to navigate the complexities of CHO DNA, CHO DNA Detection, and CHO Residual DNA Kit applications. Dive into the future of biopharmaceutical testing with BlueKit's pioneering solution, where precision meets efficiency.
|
Specification
|
50 Reactions.
|
Standard curve
|
|
Datasheet
|
Our innovative kit, offering compatibility with qPCR platforms, comes with 50 reactions per package, providing not just unparalleled accuracy but also cost-effectiveness for laboratories of all sizes. The Mycoplasma DNA Detection Kit (qPCR)-ZY002 is tailored for those who seek efficiency without compromising on precision. Whether for routine quality control, research, or development purposes, this kit delivers consistent results, ensuring that your products meet the stringent standards for CHO residual DNA contamination. Embedded within this product is the essence of BlueKit's dedication to supporting the biopharmaceutical industry's push towards safer, more reliable products. By choosing the Mycoplasma DNA Detection Kit (qPCR)-ZY002, you're not just acquiring a product; you're empowering your research and production with a tool designed to navigate the complexities of CHO DNA, CHO DNA Detection, and CHO Residual DNA Kit applications. Dive into the future of biopharmaceutical testing with BlueKit's pioneering solution, where precision meets efficiency.
{{item.c_type}}
{{item.title}}
{{item.c_time_limit}}
{{item.title}}
Number
Overview
Protocols
Specifications
Shipping & Returns
Video Recording
Cat.No. HG-ZY002 $1,508.00
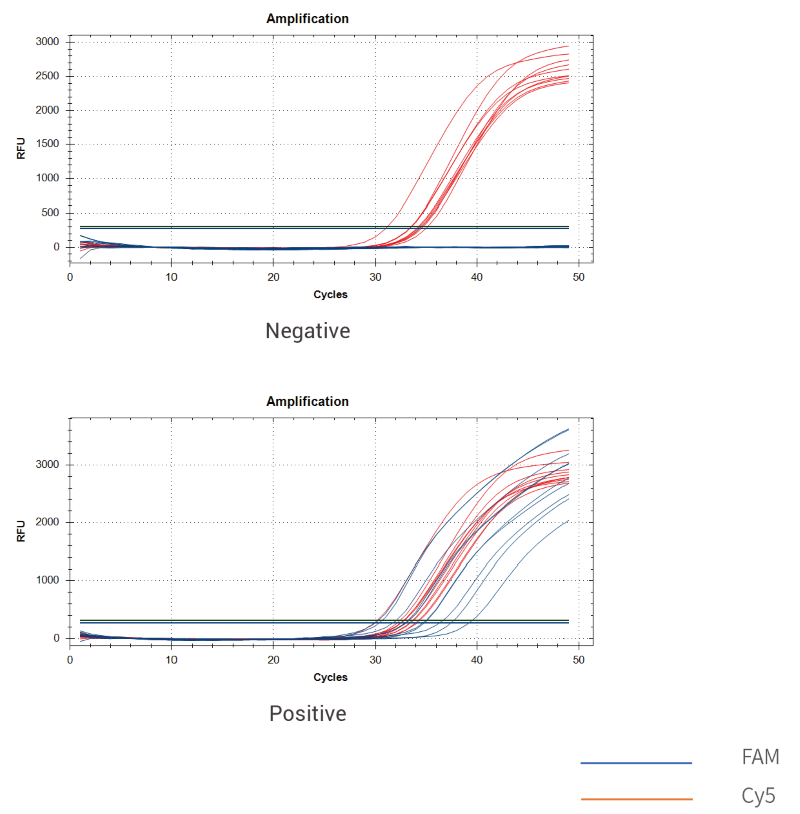
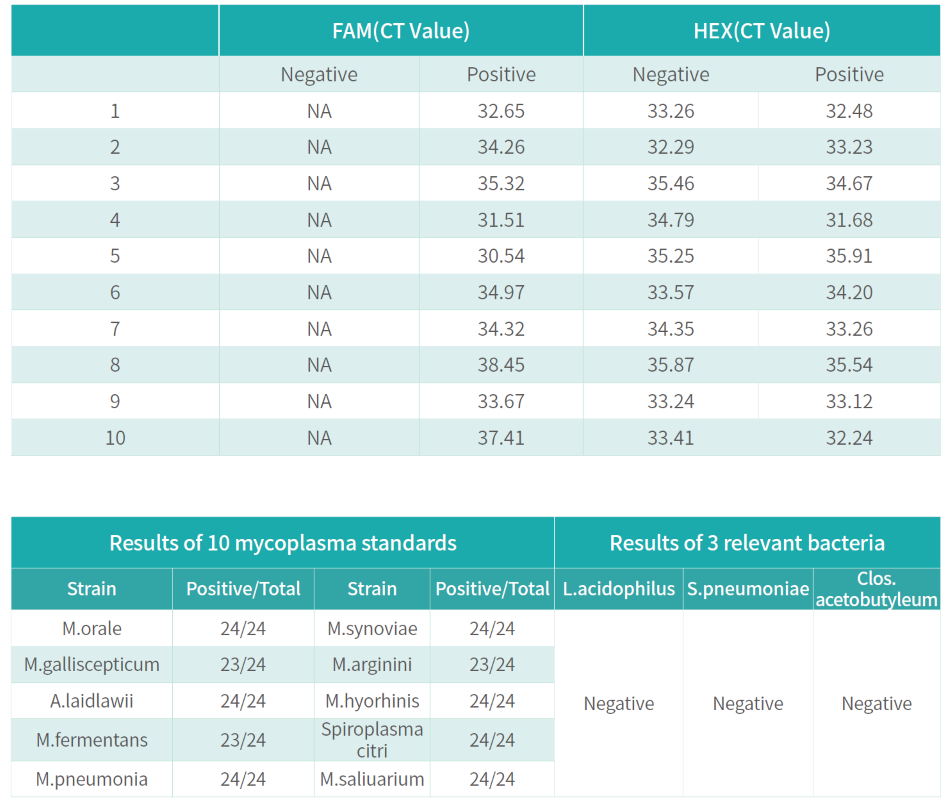
The kit is used to qualitatively detect the presence of mycoplasma contamination in master cell banks, working cell banks, virus seed lots, control cells, and cells for clinical therapy.
The kit uses qPCR-fluorescent probe technology to verify with reference to mycoplasma detectionrelated requirements in EP2.6.7 and JPXVII. It can cover more than 100 mycoplasmas and has no cross reaction with closely related strains. The detection is rapid which can be completed within 2 hours, with strong specificity.