Efficient Detection of DNA Residual | E.coli qPCR Kit | BlueKit
Efficient Detection of DNA Residual | E.coli qPCR Kit | BlueKit
$ {{single.sale_price}}
In an era where biopharmaceutical production and genetic research are advancing rapidly, ensuring the purity and safety of biological products is paramount. BlueKit's E.coli Residual DNA Detection Kit (qPCR) stands at the forefront of facilitating this crucial need, offering an unrivaled solution for detecting and quantifying DNA residuals in biological samples. This innovative product leverages the precision of quantitative Polymerase Chain Reaction (qPCR) technology to offer researchers and professionals a reliable, efficient, and highly sensitive tool for the assessment of contaminant DNA.
The presence of residual DNA from host organisms, such as E.coli, in biopharmaceutical products, can pose significant safety concerns, including potential immunogenic reactions in recipients. Thus, regulatory authorities worldwide have set stringent limits on the allowable levels of DNA residuals in therapeutic products. The BlueKit E.coli Residual DNA Detection Kit is designed to meet and exceed these regulatory demands, providing a robust standard curve that ensures the accurate quantification of even minute quantities of DNA residual. This level of precision is vital for both adherence to safety standards and the integrity of scientific research. Beyond its advanced technical capabilities, the kit is engineered for user convenience. It simplifies the complex process of qPCR, making it accessible even to those with limited molecular biology experience. Each component of the kit is pre-optimized, reducing the potential for human error and increasing the reproducibility of results across different batches and operators. With BlueKit's solution, laboratories and companies can not only ensure compliance with safety regulations but also foster trust in their products among stakeholders and end-users by demonstrating commitment to the highest safety and quality standards.
|
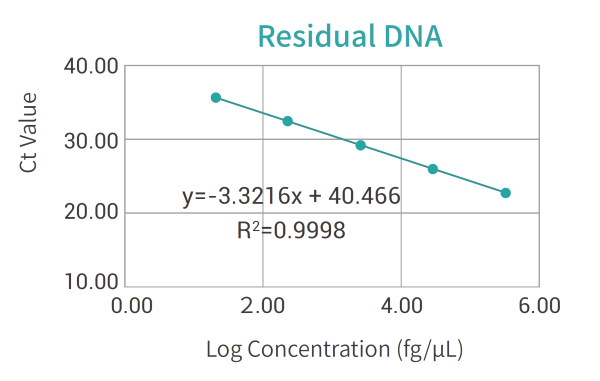
Standard curve
|
|
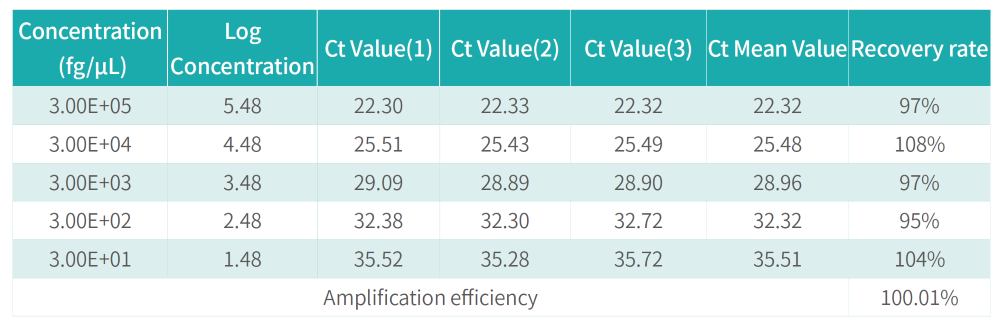
Datasheet
|
The presence of residual DNA from host organisms, such as E.coli, in biopharmaceutical products, can pose significant safety concerns, including potential immunogenic reactions in recipients. Thus, regulatory authorities worldwide have set stringent limits on the allowable levels of DNA residuals in therapeutic products. The BlueKit E.coli Residual DNA Detection Kit is designed to meet and exceed these regulatory demands, providing a robust standard curve that ensures the accurate quantification of even minute quantities of DNA residual. This level of precision is vital for both adherence to safety standards and the integrity of scientific research. Beyond its advanced technical capabilities, the kit is engineered for user convenience. It simplifies the complex process of qPCR, making it accessible even to those with limited molecular biology experience. Each component of the kit is pre-optimized, reducing the potential for human error and increasing the reproducibility of results across different batches and operators. With BlueKit's solution, laboratories and companies can not only ensure compliance with safety regulations but also foster trust in their products among stakeholders and end-users by demonstrating commitment to the highest safety and quality standards.
{{item.c_type}}
{{item.title}}
{{item.c_time_limit}}
{{item.title}}
Overview
Protocols
Specifications
Shipping & Returns
Video Recording
Cat.No. HG-ED001 $1,508.00
This kit is designed for the quantitative detection of E.coli host cell DNA in intermediates, semifinished products and finished products of various biological products.
This kit adopts the principle of Taqman probe to quantitatively detect E.coli residual DNA in samples.
The kit is a rapid, specific and reliable device, with the minimum detection limit reaching fg level.
| Performance |
Assay range |
|
|
Limit of quantitation |
|
|
|
Limit of detection |
|
|
|
Precision |
|