Cdmo Services pro lentiviral vectors - Commercial gradu
SERVITIUM
| CDmo Services pro lentiviral vectors (Hilenti® Platform) | ||||
| Genimen | SERVITIUM | |||
| Commercial gradu | 1 | GMP vestibulum Lentiviral vectors |
● Biooreactor Processus: V ~ L Disposable Biooreactor Processus (Subject to customized mutationes) ● Productio scale: II ~ XXX L (Subject to customized mutationes) |
/ |
Commoda
| Utens nostris suggestus de Serum - Free Suspension culturing of Lentiviral Vectors:
• libera est animal - derived components in processus • linearly scaled sursum productio de lentiviral vectors • Using unum continens L l PROMPTU BIOREACTOR • celle ripam creaturae in separatum officinas • Dispensing Final Products usura sterilis Isolator • Dedicavit lentivirus ratio pro currus - T cellulis, cum princeps infectio efficientiam • humilis productio costs et probatio costs (non requiruntur temptationis pro BSA et RELICTUM pancreatic enzymes) • Plures prospere ind penmissionibus ad NMPA de lentiviral vectors ad currus - T cellulis |
Processu

Imperium
| Productio | Test item | Test modus |
| Harvest fluidum | Adventitia virus contagione | Modus MMMCCCII de CHP MMXX |
| Replication - competenti lentiviruses | Indicator cell culturae modum | |
| Medicamento Substantia / Complevit Product | Species | Visual inspectionem |
| Sterilitas | Modus MCI de CHP MMXX | |
| Mycoplasma |
Modus MMMCCCI de CHP MMXX |
|
| pH | Modus DCXXXI de CHP MMXX | |
| Osmolality | Modus DCXXXII de CHP MMXX | |
| Target gene structuram idem | Sequencing | |
| RELICTUM exercitum cellula dapibus | Elisa | |
| Corporalis Titter (P24) | Elisa | |
| Titter muneris | Fluunt Cytometry | |
| Endotoxin | Modus MCXLIII de CHP MMXX | |
| RELICTUM Benzonase | Elisa | |
| RELICTUM Hostel Cell DNA | Q - PCR | |
| RELICTUM E1A Gene Transfer | Co - Methodus Culture | |
| RELICTUM SV40 Gene translatio | Co - Methodus Culture |
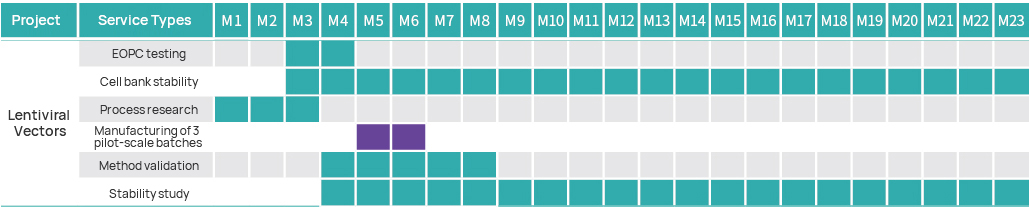
Project Timeline
Project Management Plan
Hebgene Project Management Team, constans chishistae, project managers, project QA et GMP peritorum, et faciet studium ut lenis et sonus operationem et singulis et omni GMP project.